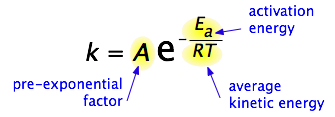
The Arrhenius equation is a mathematical relationship that describes the effect of temperature on the rate of chemical reactions. It was first proposed by the Swedish chemist Svante Arrhenius in 1889. The equation is given by:
where k is the rate constant of the reaction, A is the pre-exponential factor (or frequency factor), Ea is the activation energy of the reaction, R is the gas constant, and T is the absolute temperature.
The Arrhenius equation shows that the rate of a chemical reaction increases as the temperature increases. This is because at higher temperatures, the reactant molecules have more kinetic energy, which increases the frequency of their collisions and the likelihood of successful collisions leading to product formation.
The pre-exponential factor A represents the frequency of collisions between reactant molecules, and is independent of temperature. The activation energy Ea represents the minimum energy required for a successful collision between reactant molecules to lead to product formation, and is a measure of the reaction’s barrier to progress.
The Arrhenius equation has important applications in fields such as chemical kinetics, industrial chemistry, and materials science. It can be used to predict the rate of chemical reactions at different temperatures, optimize reaction conditions for industrial processes, and understand the temperature dependence of material properties such as diffusion coefficients, viscosity, and conductivity.
 (909) 987-1774
(909) 987-1774 Email Us
Email Us